View review activity
You can see all activities for a review across versions, including when reviews were opened, closed, when new versions were published, or when signatures were added. This can be useful for collecting electronic signature information for FDA 21 CFR Part 11 compliance.
You can also see item progress and participant progress for each version of the review.
To see review activity:
From the review with activities you want to view, select Stats.
In the page that opens, select the Review activity tab.
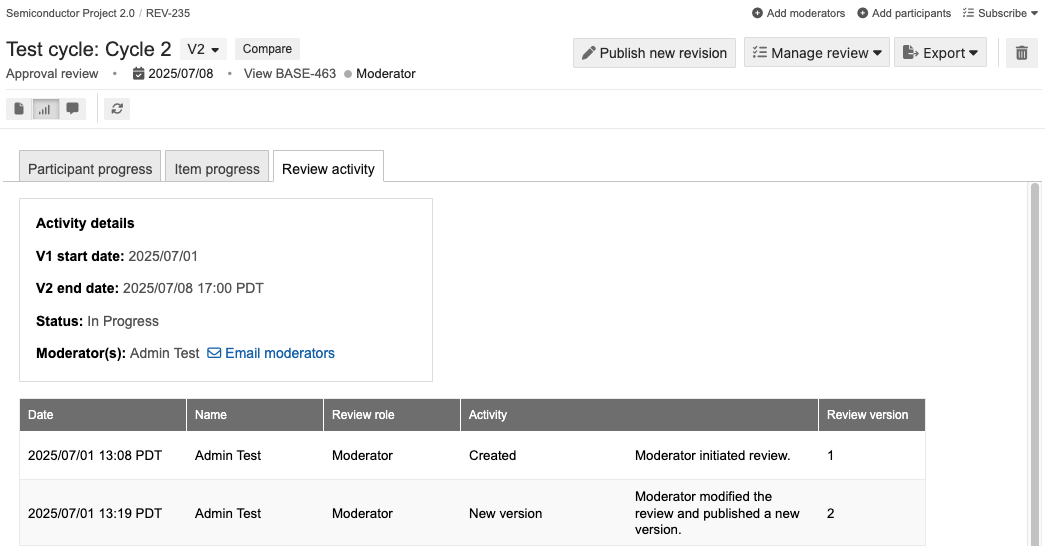
The table shows activity for all versions since the review was created.